
Dr. Peter Ward explains at The Hill Greenhouse gases simply do not absorb enough heat to warm Earth Excerpts in italics with my bolds.
Science is not done by consensus, by popular vote, or by group think. As Michael Crichton put it: “In science consensus is irrelevant. What is relevant is reproducible results. The greatest scientists in history are great precisely because they broke with the consensus.”
The drive to demonstrate scientific consensus over greenhouse-warming theory has had the unintended consequence of inhibiting genuine scientific debate about the ultimate cause of global warming.
Believers of “the consensus” argue that anyone not agreeing with them is uninformed, an idiot or being paid by nefarious companies. The last thing most climate scientists want to consider at this point, when they think they are finally winning the climate wars, is the possibility of some problem with the science of greenhouse-warming theory. Believe me, I have tried for several years to communicate the problem to numerous leading climate scientists.
New data and improved understanding now show that there is a fatal flaw in greenhouse-warming theory. Simply put: greenhouse gases do not absorb enough of the heat radiated by Earth to cause global warming.
Understanding this very surprising and rather blunt statement is much easier than you might think. It gets down to understanding why a traditional light bulb gives off a great deal of heat whereas a new LED light bulb producing the same amount of light remains quite cool.
Heat is what makes us feel warm. More formally, heat is thermal energy flowing spontaneously from a warmer body to a cooler body. Thermal energy is well observed at the molecular level to be the oscillation of all the bonds that hold matter together. The hotter the body of matter, the higher the frequencies of oscillation and the higher the amplitudes of oscillation at each frequency of oscillation. In this way, heat and the temperature that results from absorbing heat both consist of a very broad spectrum of all of these frequencies of oscillation.
A traditional light bulb uses a large amount of electricity to heat the tungsten filament to temperatures around 5500 degrees, causing the filament to glow white hot. This high temperature is required to produce visible white light. The glowing filament gives off a very broad spectrum of frequencies of radiation, however, that we perceive as heat. Only a very small number of the highest of these frequencies are useful as visible light.
A new LED light bulb, on the other hand, uses a very small amount of electricity to cause a diode to emit a very narrow range of frequencies within the spectrum of visible light. The LED radiates only visible light — it does not radiate heat.

If you look at the LED with an infrared camera, you can see just where it gets hot. The hottest part is the base of the bulb where there is an AC to DC converter which is the primary source of heat for this bulb. For the incandescent bulbs, the hottest part is the top of the bulb.
The primary purpose of a light bulb is to provide visible light. To repeat, a traditional light bulb radiates heat, a small portion of which is visible light. An LED on the other hand, only radiates visible light, requiring much less electricity. This is why you can substantially reduce your electric bills by replacing traditional incandescent light bulbs with LED light bulbs.
How does this apply to greenhouse gases?
Detailed laboratory studies of absorption of radiation show that carbon dioxide absorbs less than 16 percent of all the frequencies making up the heat radiated by Earth. Just like LEDs, this limited number of frequencies absorbed by carbon dioxide does not constitute heat. This limited number of frequencies cannot cause an absorbing body of matter to get much hotter because it contains only a very small part of the heat required to do so.
Current radiation theory and current climate models assume that all radiation is created equal—that all radiation is the same no matter the temperature of the radiating body. Current theory simply assumes that what changes is the amount of such generic radiation measured in watts per square meter.
Extensive observations of radiation emitted by matter at different temperatures, however, show us clearly that the physical properties of radiation, the frequencies and amplitudes of oscillation making up radiation, increase in value rapidly with increasing temperature of the radiating body.
Climate scientists argue that the thermal energy absorbed by greenhouse gases is re-radiated, causing warming of air, slowing cooling of Earth and even directly warming Earth.
There simply is not enough heat involved in any of these proposed processes to have any significant effect on global warming. Greenhouse-warming theory “just ain’t so.”
 Peter L. Ward worked 27 years with the United States Geological Survey. He was the chairman of the White House Working Group on Natural Disaster Information Systems during the Clinton administration. He’s published more than 50 scientific papers. He retired in 1998 but continues working to resolve several enigmatic observations related to climate change. His work is described in detail at WhyClimateChanges.com and in his book What Really Causes Global Warming? Greenhouse gases or ozone depletion? Follow him on Twitter at @yclimatechanges.
Peter L. Ward worked 27 years with the United States Geological Survey. He was the chairman of the White House Working Group on Natural Disaster Information Systems during the Clinton administration. He’s published more than 50 scientific papers. He retired in 1998 but continues working to resolve several enigmatic observations related to climate change. His work is described in detail at WhyClimateChanges.com and in his book What Really Causes Global Warming? Greenhouse gases or ozone depletion? Follow him on Twitter at @yclimatechanges.
Comment:
The above article is an opinion piece that does not go deeply into the scientific case underlying the conclusions. That analysis can be read at Ward’s paper Why greenhouse-warming theory is physically impossible
Overview
Thus greenhouse-warming theory is based on the assumption that (1) radiative energy can be quantified by a single number of watts per square meter, (2) the assumption that these radiative forcings can be added together, and (3) the assumption that Earth’s surface temperature is proportional to the sum of all of these radiative forcings. A fundamentally new understanding of the physics of thermal energy and the physics of heat, described below, shows that all three assumptions are mistaken. There are other serious problems: (4) greenhouse gases absorb only a small part of the radiation emitted by Earth, (5) they can only reradiate what they absorb, (6) they do not reradiate in every direction as assumed, (7) they make up only a tiny part of the gases in the atmosphere, and (8) they have been shown by experiment not to cause significant warming. (9) The thermal effects of radiation are not about amount of radiation absorbed, as currently assumed, they are about the temperature of the emitting body and the difference in temperature between the emitting and the absorbing bodies as described below.
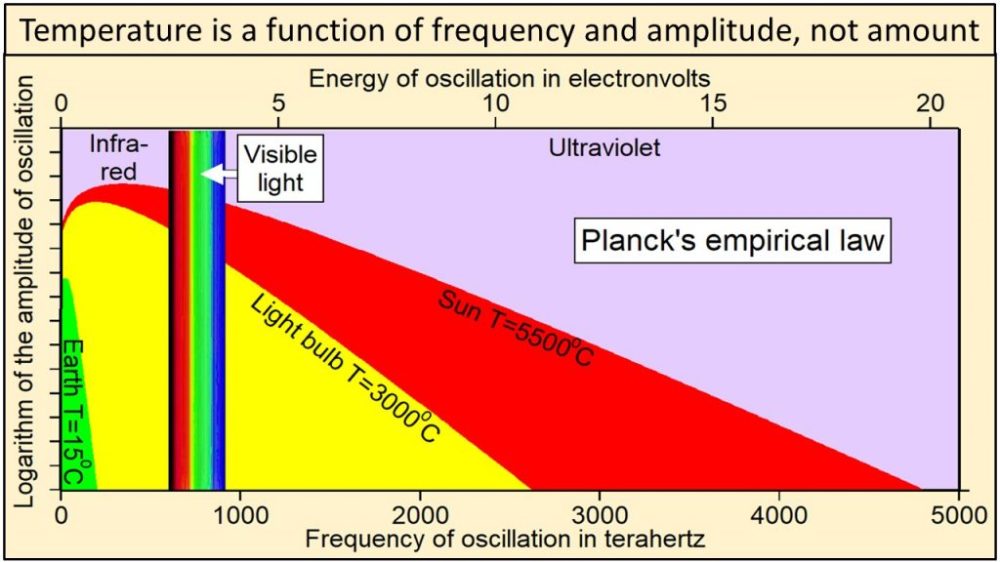
Thermal radiation from Earth, at a temperature of 15 oC, consists of the narrow continuum of frequencies of oscillation shown in green in this plot of Planck’s empirical law. Thermal radiation from the tungsten filament of an incandescent light bulb at 3000 oC consists of a broader continuum of frequencies shown in yellow and green. Thermal radiation from Sun at 5500 oC consists of a much broader continuum of frequencies shown in red, yellow and green.
Note in this plot of Planck’s empirical law that the higher the temperature, 1) the broader the continuum of frequencies, 2) the higher the amplitude of oscillation at each and every frequency, and 3) the higher the frequencies of oscillation that are oscillating with the largest amplitudes of oscillation. Radiation from Sun shown in red, yellow, and green clearly contains much higher frequencies and amplitudes of oscillation than radiation from Earth shown in green. Planck’s empirical law shows unequivocally that the physical properties of radiation are a function of the temperature of the body emitting the radiation.
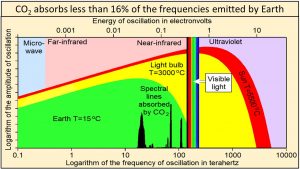
Ångström (1900) showed that “no more than about 16 percent of earth’s radiation can be absorbed by atmospheric carbon dioxide, and secondly, that the total absorption is very little dependent on the changes in the atmospheric carbon dioxide content, as long as it is not smaller than 0.2 of the existing value.” Extensive modern data agree that carbon dioxide absorbs less than 16% of the frequencies emitted by Earth shown by the vertical black lines of this plot of Planck’s empirical law where frequencies are plotted on a logarithmic x-axis. These vertical black lines show frequencies and relative amplitudes only. Their absolute amplitudes on this plot are arbitrary.
Temperature at Earth’s surface is the result of the broad continuum of oscillations shown in green. Absorbing less than 16% of the frequencies emitted by Earth cannot have much effect on the temperature of anything.
Update January 17, 2020
Dr. Ward’s journey of discovery is provided here: CO2, SO2, O3: A journey of Discovery
Reblogged this on Climate Collections.
LikeLike
Reblogged this on Tallbloke's Talkshop and commented:
To go to the technical discussion, search for ‘Overview’.
LikeLike
Yesterday I bought a couple of 10 W LED bulbs to replace my not so wonderful mercury vapour ones that were supposed to last for ever and boy are they bright! Hopefully they will last for a while but at £2.50 each it seems a bargain.
LikeLike
Adrian, I was alerted to this issue when we moved to a new apartment where the closet light fixtures were limited to 60W bulbs. The illumination was inadequate, but replacing them with 15W LEDs gives lighting equivalent to 100W traditional bulbs.
LikeLike
It comes down to this:1) All radiation spectra are not “heat”. 2) The small quantity of CO2 and other greenhouse gases in the atmosphere would have to be heated to a tremendously high temperature to account for any significant amount of “global warming”–to my knowledge a like phenomenon has never been observed. 3) Attributing global temperature anomalies to 415 ppm CO2 is non-scientific, un-provable and manipulative! The position is a launching pad to punish the population with excessive taxes and control their behavior and mobility.
LikeLike