
When the Raman effect came up last year in relation to GHGs (Greenhouse Gases), I was firstly confused thinking it was talk of asian noodles. So I have had to learn more, and while the effect is real and useful, I doubt it is a factor concerning global warming/climate change. This post provides information principally from two sources consistent with many others I read.
One article is Raman Spectroscopy from University of Pennsylvania. Excerpts in italics with my bolds.
Raman Effect
Raman spectroscopy is often considered to be complementary to IR spectroscopy. For symmetrical molecules with a center of inversion, Raman and IR are mutually exclusive. In other words, bonds that are IR-active will not be Raman-active and vice versa. Other molecules may have bonds that are either Raman-active, IR-active, neither or both.
Raman spectroscopy measures the scattering of light by matter. The light source used in Raman spectroscopy is a laser.
The laser light is used because it is a very intense beam of nearly monochromatic light that can interact with sample molecules. When matter absorbs light, the internal energy of the matter is changed in some way. Since this site is focused on the complementary nature of IR and Raman, the infrared region will be discussed. Infrared radiation causes molecules to undergo changes in their vibrational and rotational motion. When the radiation is absorbed, a molecule jumps to a higher vibrational or rotational energy level. When the molecule relaxes back to a lower energy level, radiation is emitted. Most often the emitted radiation is of the same frequency as the incident light. Since the radiation was absorbed and then emitted, it will likely travel in a different direction from which it came. This is called Rayleigh scattering. Sometimes, however, the scattered (emitted) light is of a slightly different frequency than the incident light. This effect was first noted by Chandrasekhara Venkata Raman who won the Nobel Prize for this discovery. (6) The effect, named for its discoverer, is called the Raman effect, or Raman scattering.
Raman scattering occurs in two ways. If the emitted radiation is of lower frequency than the incident radiation, then it is called Stokes scattering. If it is of higher frequency, then it is called anti-Stokes scattering.
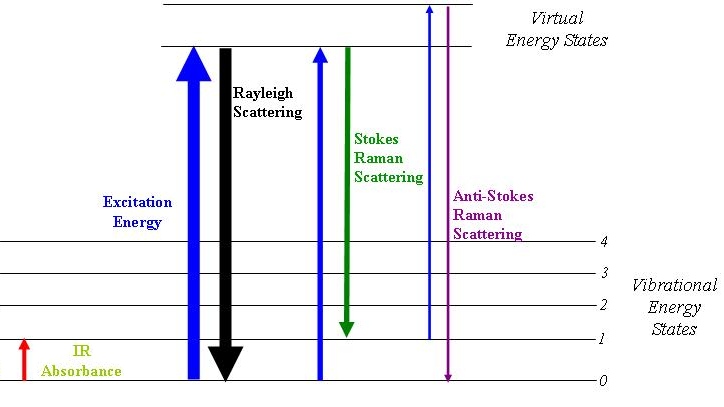
Energy Diagram Scattering (Source: Wikipedia)
The Blue arrow in the picture to the left represents the incident radiation. The Stokes scattered light has a frequency lower than that of the original light because the molecule did not relax all the way back to the original ground state. The anti-Stokes scattered light has a higher frequency than the original because it started in an excited energy level but relaxed back to the ground state.
Though any Raman scattering is very low in intensity, the Stokes scattered radiation is more intense than the anti-Stokes scattered radiation.
The reason for this is that very few molecules would exist in the excited level as compared to the ground state before the absorption of radiation. The diagram shown represents electronic energy levels as shown by the labels “n=”. The same phenomenon, however, applies to radiation in any of the regions.
Another article is Raman Techniques: Fundamentals and Frontiers by Robin R. Jones et al. at 2019 at US National Library of Medicine.
Abstract
Driven by applications in chemical sensing, biological imaging and material characterisation, Raman spectroscopies are attracting growing interest from a variety of scientific disciplines. The Raman effect originates from the inelastic scattering of light, and it can directly probe vibration/rotational-vibration states in molecules and materials.
Despite numerous advantages over infrared spectroscopy, spontaneous Raman scattering is very weak, and consequently, a variety of enhanced Raman spectroscopic techniques have emerged.
These techniques include stimulated Raman scattering and coherent anti-Stokes Raman scattering, as well as surface- and tip-enhanced Raman scattering spectroscopies. The present review provides the reader with an understanding of the fundamental physics that govern the Raman effect and its advantages, limitations and applications. The review also highlights the key experimental considerations for implementing the main experimental Raman spectroscopic techniques. The relevant data analysis methods and some of the most recent advances related to the Raman effect are finally presented. This review constitutes a practical introduction to the science of Raman spectroscopy; it also highlights recent and promising directions of future research developments.
Fundamental Principles
When light interacts with matter, the oscillatory electro-magnetic (EM) field of the light perturbs the charge distribution in the matter which can lead to the exchange of energy and momentum leaving the matter in a modified state. Examples include electronic excitations and molecular vibrations or rotational-vibrations (ro-vibrations) in liquids and gases, electronic excitations and optical phonons in solids, and electron-plasma oscillations in plasmas [108].
Spontaneous Raman
When an incident photon interacts with a crystal lattice or molecule, it can be scattered either elastically or inelastically. Predominantly, light is elastically scattered (i.e. the energy of the scattered photon is equal to that of the incident photon). This type of scattering is often referred to as Rayleigh scattering. The inelastic scattering of light by matter (i.e. the energy of the scattered photon is not equal to that of the incident photon) is known as the Raman effect [1, 4, 6]. This inelastic process leaves the molecule in a modified (ro-)vibrational state
In the case of spontaneous Raman scattering, the Raman effect is very weak; typically, 1 in 10^8 of the incident radiation undergoes spontaneous Raman scattering [6].
The transition from the virtual excited state to the final state can occur at any point in time and to any possible final state based on probability. Hence, spontaneous Raman scattering is an incoherent process. The output signal power is proportional to the input power, scattered in random directions and is dependent on the orientation of the polarisation. For example, in a system of gaseous molecules, the molecular orientation relative to the incident light is random and hence their polarisation wave vector will also be random. Furthermore, as the excited state has a finite lifetime, there is an associated uncertainty in the transition energy which leads to natural line broadening of the wavelength as per the Heisenberg uncertainty principle (∆E∆t ≥ ℏ/2) [1]. The scattered light, in general, has polarisation properties that differ from that of the incident radiation. Furthermore, the intensity and polarisation are dependent on the direction from which the light is measured [1]. The scattered spectrum exhibits peaks at all Raman active modes; the relative strength of the spectral peaks are determined by the scattering cross-section of each Raman mode [108]. Photons can undergo successive Rayleigh scattering events before Raman scattering occurs as Raman scattering is far less probable than Rayleigh scattering.
Laser Empowered Raman Scattering
Coherent light-scattering events involving multiple incident photons simultaneously interacting with the scattering material was not observed until laser sources became available in the 1960s, despite predictions being made as early as the 1930s [37, 38]. The first laser-based Raman scattering experiment was demonstrated in 1961 [39]. Stimulated Raman scattering (SRS) and CARS have become prominent four-wave mixing techniques and are of interest in this review.
SRS is a coherent process providing much stronger signals relative to spontaneous Raman spectroscopy as well as the ability to time-resolve the vibrational motions.
Raman is generally a very weak process; it is estimated that approximately one in every 10^8 photons undergo Raman scattering spontaneously [6]. This inherent weakness poses a limitation on the intensity of the obtainable Raman signal. Various methods can be used to increase the Raman throughput of an experiment, such as increasing the incident laser power and using microscope objectives to tightly focus the laser beam into small areas. However, this can have negative consequences such as sample photobleaching [139]. Placing the analyte on a rough metal surface can provide orders of magnitude enhancement of the measured Raman signal, i.e. SERS.
Summary
It seems to me that spontaneous scattering is the only possible way that the Raman effect could influence the radiative profile of the atmosphere. Sources like those above convince me that lacking laser intensity, natural light does not produce a Raman effect in the air of any significance for it to be considered a climate factor.

Thanks for the review. I’m surprised I remembered bits and pieces from a survey course I had about 50 years ago. The 1970’s era instrument NC State had took up a fair-sized lab and took quite a bit of power and “carbon footprint” to run.
LikeLike
Thanks Bob. My undergraduate studies included some work with traditional spectroscopy in quantitative chemistry, but we never heard of Raman, not even the asian noodles.
LikeLiked by 1 person
There has never been a claim the Raman effect affects the atmosphere. What the Raman spectrometer does however is exploit the Raman effect to infer the quantum predict emission spectra line that IR infrared instruments do not (always) detect. The Raman spectrometer detects N2 and O2 and all the other molecules along with their temperatures and concentrations.
LikeLike
Here is an article I wrote a year or two back. https://principia-scientific.org/settling-the-climate-woo-with-quantum-based-raman-spectroscopy/
LikeLike
Blair, thanks for commenting and for your continuing efforts in this line of research. I was dismissive because I have yet to connect the dots how the energy level is significant enough to matter for planetary heat transfers. Maybe your understanding can help me.
I did post five years ago on N2 radiation, based upon this article:
Click to access nph-iarticle_query
I’d be interested in your reaction to it. I also have read papers by Peter Ward regarding atmospheric radiation. His website:
https://whyclimatechanges.com/impossible/
And, of course, we know that satellite estimates of atmospheric temperatures (like UAH) rely on microwave readings of oxygen, which are then processed. But I still don’t see how far IR emissions can be a climate factor. My paradigm has been that O2 and N2 contribute to our mild earth climate by not emitting IR, and indeed must pass energy to IR-active gases at TOA for escape into space. I am not yet persuaded otherwise.
LikeLiked by 1 person
Hello Ron, it is certainly, at least for me, a perplexing issue. On one hand (the thermoelectric blackbody curves) N2 and O2 are assumed not to participate, and on the other, Raman observation of their quantum modes in the infrared revealing their temperatures and concentrations, they seem to. I do not know what to make of the Ward paper: I will need to take a closer look at his methodology. If I see a hint of thermoelectrics used I will be more satisfied on how he came to his conclusions. I will look at it. I have recently conducted multiple experiments on the thermoelectric detectors and am currently writing these up. Things are certainly not what we think they are. I think they have totally mislead us. My updates will be on my blog. Regards.
LikeLike
Thanks Blair. I look forward to your posts. i suppose the most complete explanation of my conclusions about climate change/global warming is written in a post called Climate Reductionism.
LikeLiked by 1 person